



Applications | Structured Light Visible Micro-Imaging
Release time:Release time:2024 - 02 - 23
Structure Illumination Microscopy (SIM) was first developed and proposed by Mats Gustafsson in 2005, and its basic principle is based on the Moire pattern, an effect that is commonly used to produce optical illusions. The basic principle is based on the Moire pattern, an effect commonly used to create optical illusions.
Moire pattern: An optical stripe formed by the superposition of two periodic grating patterns of similar spatial frequency. When two lines or two objects interfere with each other at a constant angle and frequency, and the human eye cannot distinguish between the two lines or two objects, only the pattern of interference can be seen.
When two small-sized (high-frequency) grids are superimposed overlaying the rightmost image, the interference between them displays a larger-sized (low-frequency) grid that will contain information from the two smaller grids.
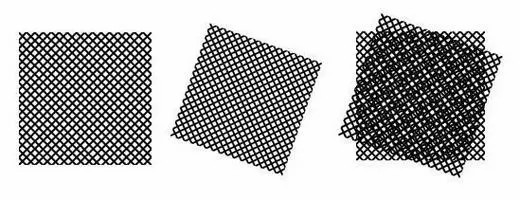
▲ Moire pattern formed by the interference of two fine meshes overlapping each other at an angle.
In practice, by inserting a structured light generating device (such as a grating, spatial light modulator, or digital micromirror array DMD, etc.) into the illumination light path, the illumination light is modulated to form a pattern of regularly varying brightness, which is then projected onto the sample via the objective lens, and the fluorescent signal generated by the modulated light is then received by the camera.
By moving and rotating the illumination pattern to cover various areas of the sample, and combining and reconstructing the captured images with software, an image of the sample can be obtained.
Structure Illumination Microscopy (SIM) combines Structure Illumination Microscopy (SIM) with conventional optical microscopy, and through the use of a special optical illumination system, it is able to achieve the purpose of removing spurious signals outside the focal plane, so as to realize the optical layer-cutting scanning of the sample, and then realize three-dimensional reconstruction.
▲ SOPTOP M-SIM6000 Structured Light Cutting Microscope
1、Quantitative, qualitative, timing and localization measurement
Automatic determination of parameters such as cell shape, circumference, area, average fluorescence intensity and number of intracellular particles. Quantitative, qualitative, timed and localized determination of the content, components and distribution of intracellular specific structures such as lysosomes, mitochondria, endoplasmic reticulum, cytoskeleton, structural proteins, DNA, RNA, enzymes and receptor molecules.


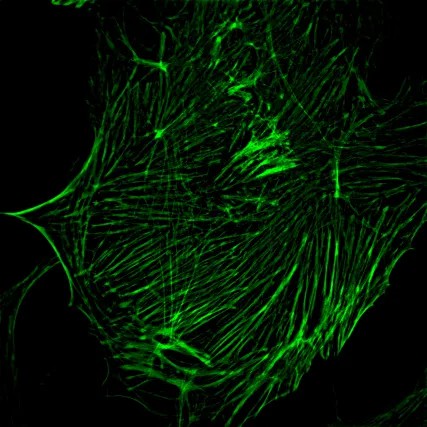
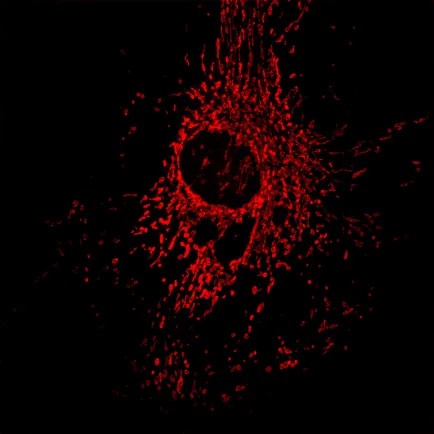
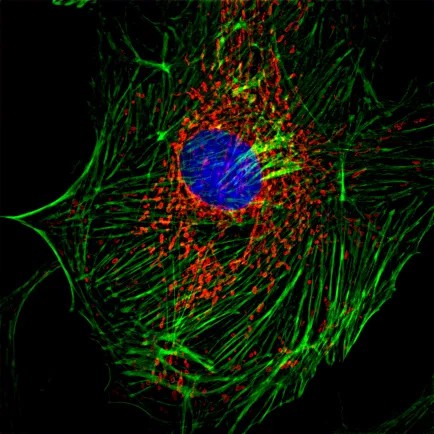
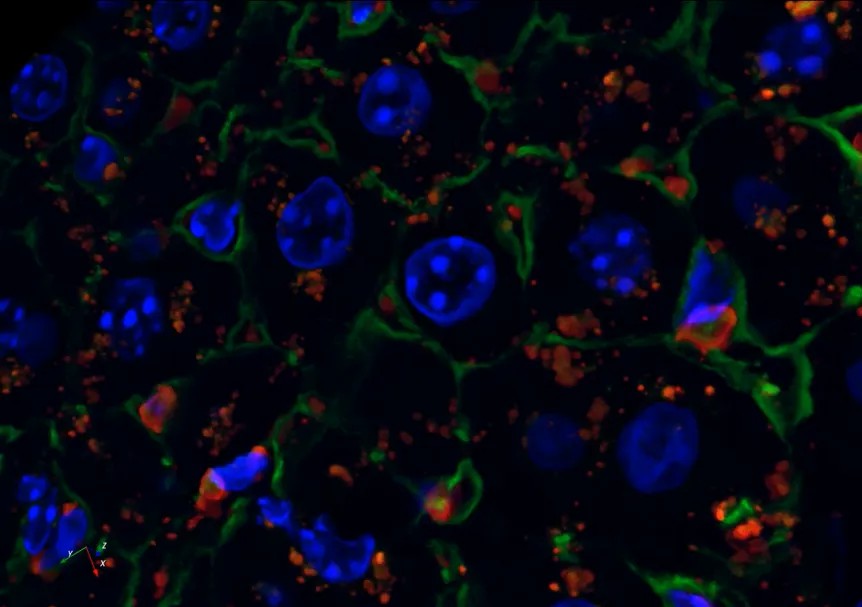
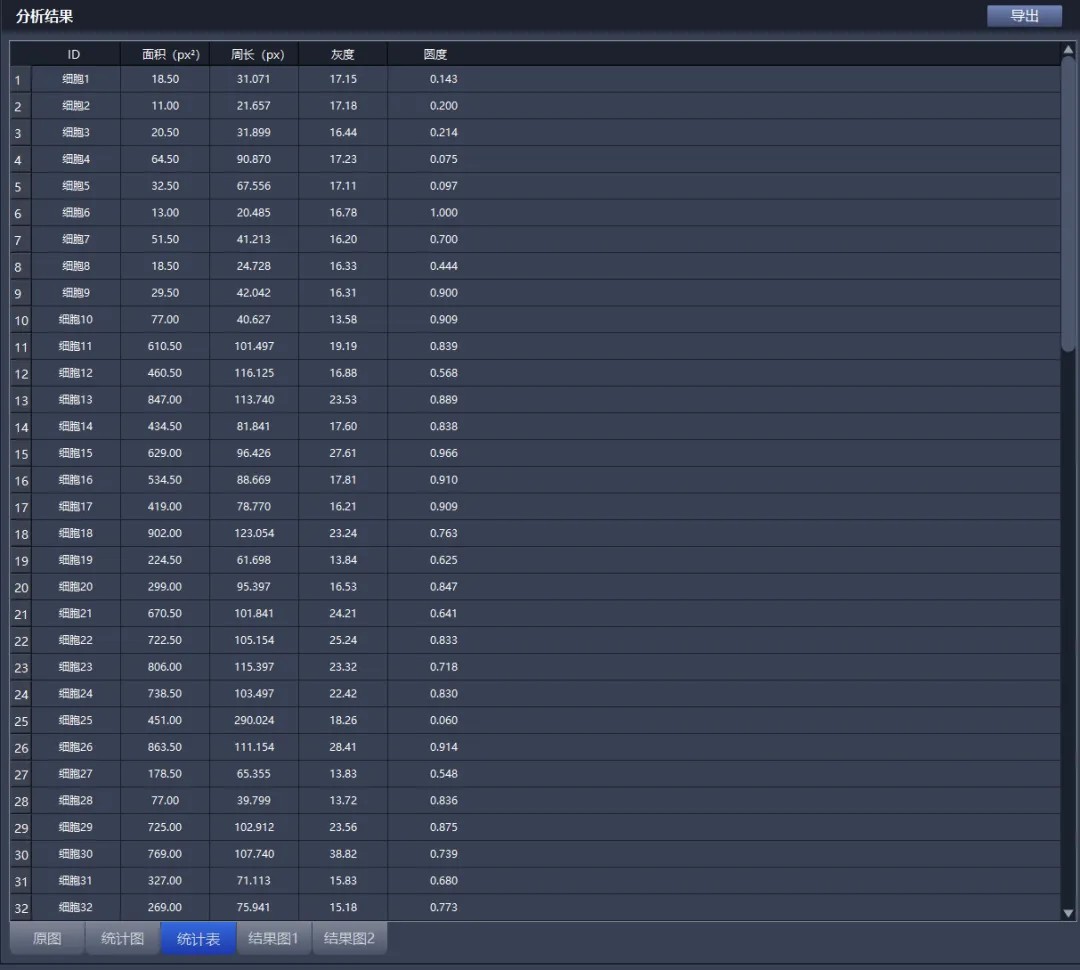
2、Three-dimensional reconstruction
The live cell line non-invasive “optical section” This function is also known as the image of the “micro CT”, the specimen can be obtained in the true sense of the three-dimensional data, computer image processing and three-dimensional reconstruction, can produce vivid and realistic three-dimensional effect, so that it can be flexible, It can be used to carry out morphological observation intuitively and reveal the spatial relationship of subcellular structure.
3、Proliferation and apoptosis of biological samples
Used for hours of long-time timing scanning, recording cell migration and growth and other cell biological phenomena.
● Embryology and developmental biology: development and signaling mechanisms in nematodes, Drosophila and zebrafish.
● Cell biology and plant biology: apoptosis, cell autophagy, cell cycle, cell metabolism, cell tracking and tracing, cytotoxicity, oxidative stress detection, phagocytosis, endocytosis, internalization of receptors, cell signaling and communication, cell motility, intracellular compartmentalization, protein synthesis and degradation, cellular and biophysical regulation.
● Yeast and bacterial research
● Stem cell research and 3D culture: image acquisition of cell proliferation and apoptosis process can be performed, which can be combined with Fluorescence Bleaching Recovery (FRAP), Fluorescence Loss in the Bleaching Process (FLIP) technology, image acquisition of cell increase and decrease changes over time, and data analysis.
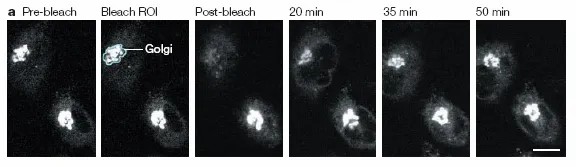
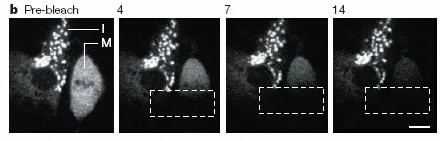
4、Intra- and extracellular communication
Fluorescent labeling of different markers can be used to track and analyze the transport and mechanism of action of ions, proteins, and other substances. It can be combined with fluorescence resonance energy transfer (FRET) to analyze the interactions between molecules. This technology can be used to study the basic mechanisms and roles of gap junction communication in embryogenesis, reproductive development, neurobiology, tumorigenesis, etc. It can also be used to identify chemicals that are potentially toxic to gap junctions.
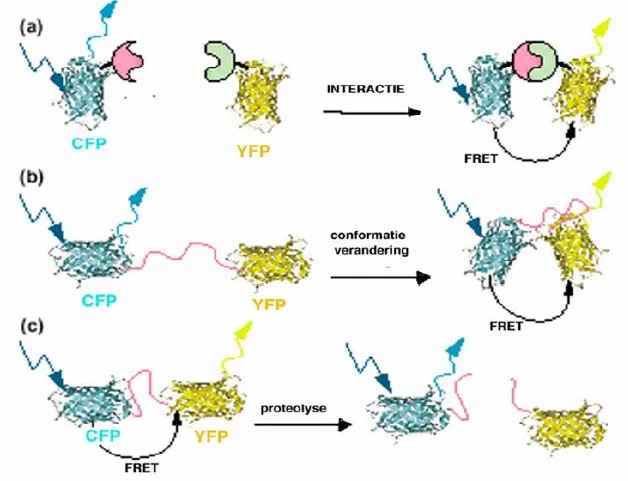
▲Applications of FRET microscopy
5、Research on fluorescent probes
Develop and test new types of fluorescent probes and synthesize new types of fluorescent probes using biochemical materials. Research on more applications of existing probes, research on existing probes that can carry more markers.
Reference:
Gadella TW Jr, van der Krogt GN, Bisseling T. GFP-based FRET microscopy in living plant cells. Trends Plant Sci. 1999 Jul;4(7):287-291. doi: 10.1016/s1360-1385(99)01426-0. PMID: 10407445.
Return list
