



Scientific Innovation | New breakthrough in hyperuricemia nephropathy, baicalin can effectively inhibit urate-induced cellular pyrolysis
Release time:Release time:2024 - 04 - 24
Hyperuricemia nephropathy is a serious complication of hyperuricemia, whose main pathological feature is the abnormally high level of uric acid in the body, with clinical manifestations such as sudden hematuria, oliguria or anuria, edema, and gout.
In this condition, the deposition of urate crystals in the kidneys causes an inflammatory response in the cells of the kidneys, leading to cell death. This new form of programmed cell death is known as pyroptosis, which is an important natural immune response of the body and plays an important role in the fight against infections.
Baicalin (BA) is a natural flavonoid extracted from the root of the traditional Chinese medicine Scutellaria baicalensis, which has a wide range of antioxidant and anti-inflammatory pharmacological activities.
This month, Prof. Lingyun Xu's group at Wuhan University of Light Industry published a study entitled “Baicalin inhibits monosodium urate crystal-induced pyroptosis in renal tubular epithelial cell line through Panx-1/P2X7 pathways: Molecular docking, molecular dynamics, and in vitro experiments” (first author: Eunice Fu) in Chem Biol Drug Des. epithelial cell line through Panx-1/P2X7 pathways: Molecular docking, molecular dynamics, and in vitro experiments” (first author: Wan-Ting Fu). Baicalin was shown to have a significant inhibitory effect on urate crystal-induced pyroptosis of renal tubular epithelial cells, highlighting its potential value in the treatment of hyperuricemic nephropathy.
The research team used urate crystals to induce inflammation and pyroptosis in renal tubular epithelial cells to mimic the inflammation and cellular damage due to high uric acid in hyperuricemia nephropathy, and then treated these cells with baicalein, while observing the cellular changes by double staining, lactate dehydrogenase (LDH) assay, and electron microscopy, and found that baicalein effectively protected the cell membranes from the urate crystals-induced damage.
In addition, the researchers assessed the expression of the pyroptosis-related genes Panx-1 and P2X7 at the mRNA and protein levels before and after treatment with baicalein, and observed the distribution and aggregation of the key proteins (GSDMD-N) in the cell membranes by SOPTOP laser confocal scanning microscopy.
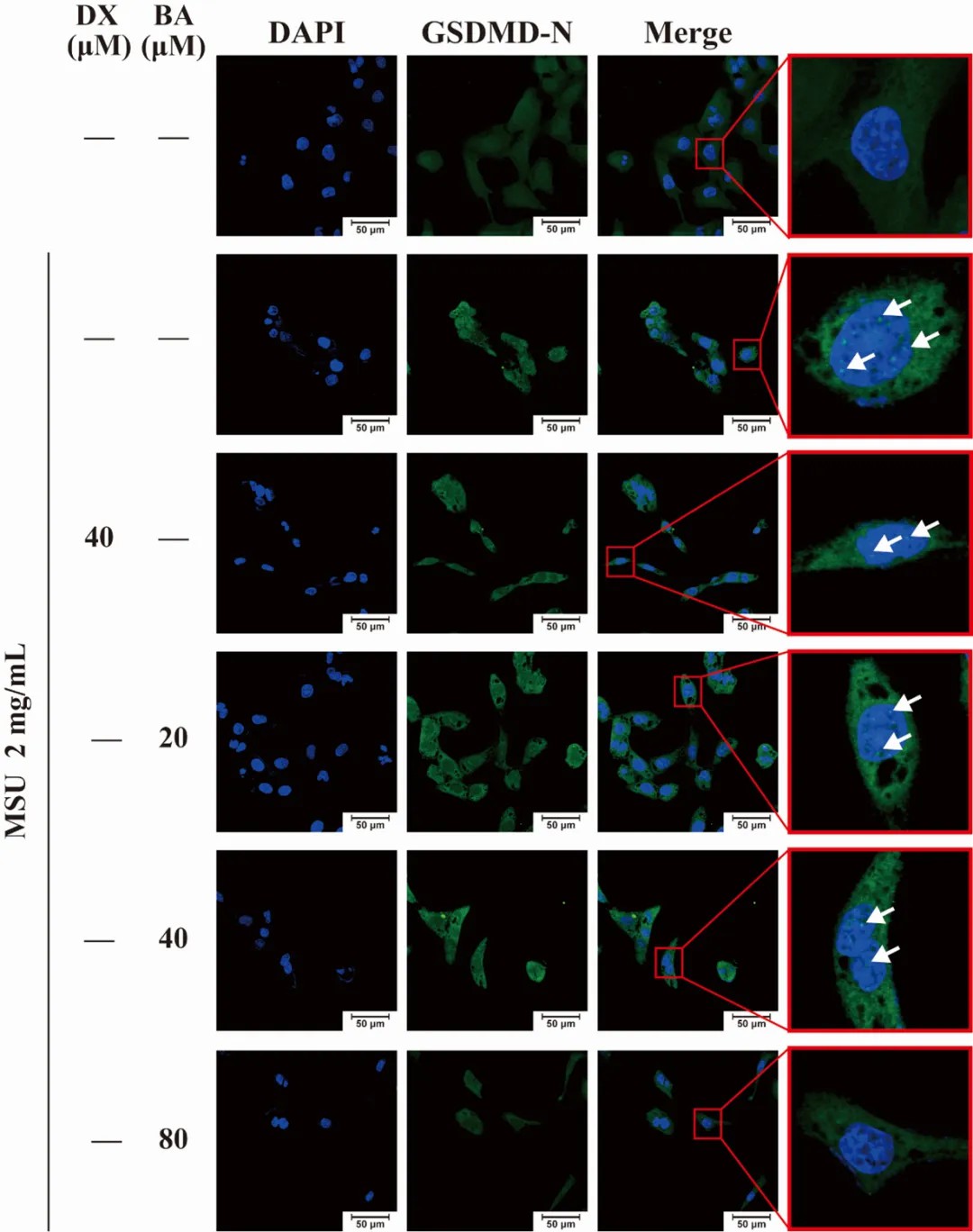
▲Immunofluorescence method to observe GSDMD protein aggregation. White arrows indicate GSDMD-N (green fluorescence); nuclei are in blue color
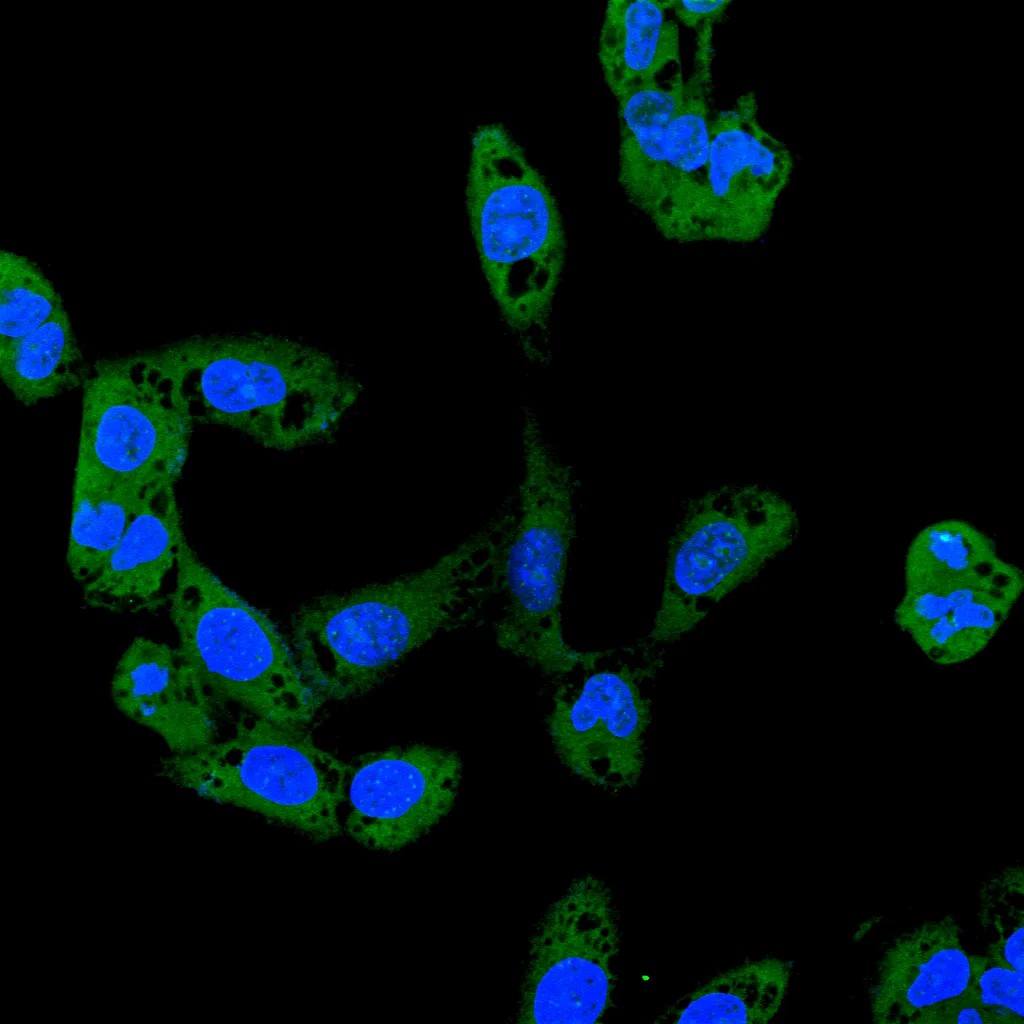
实验The results showed that there were obvious dense fluorescent dots on the cell membrane induced by urate crystals, indicating that the GSDMD-N protein was aggregated on the cell membrane; whereas the number of fluorescent dots was gradually decreased at the doses of baicalein of 20 μm and 40 μm, indicating that the protein aggregation was reduced; whereas, in the baicalein group of the high dose (80 μm), the obvious fluorescent dots could hardly be seen, which was similar to that of the normal group, which indicated that the baicalin had a good inhibitory effect on the aggregation of GSDMD-N proteins on the cell membrane.
Compared with ordinary inverted fluorescence microscopes, the SOPTOP Laser Confocal Scanning Microscope is able to minimize the interference caused by component displacement, and improve the signal-to-noise ratio and axial resolution of the image by enhancing the filtering of non-focal plane signals while ensuring the efficiency of fluorescence signal acquisition.
With the Z-Stack imaging function, images at different depths can be acquired, helping researchers to localize the exact location and aggregation status of GSDMD-N protein within the cell.
Thesis Information:
Fu W, Liu Z, Wang Y, Li X, Yu X, Li Y, Yu Z, Qiu Y, Mei Z, Xu L. Baicalin inhibits monosodium urate crystal-induced pyroptosis in renal tubular epithelial cell line through Panx-1/P2X7 pathways: Molecular docking, molecular dynamics, and in vitro experiments. Chem Biol Drug Des. 2024 Apr;103(4):e14522.
doi: 10.1111/cbdd.14522. PMID: 38580458.
Return list
